-
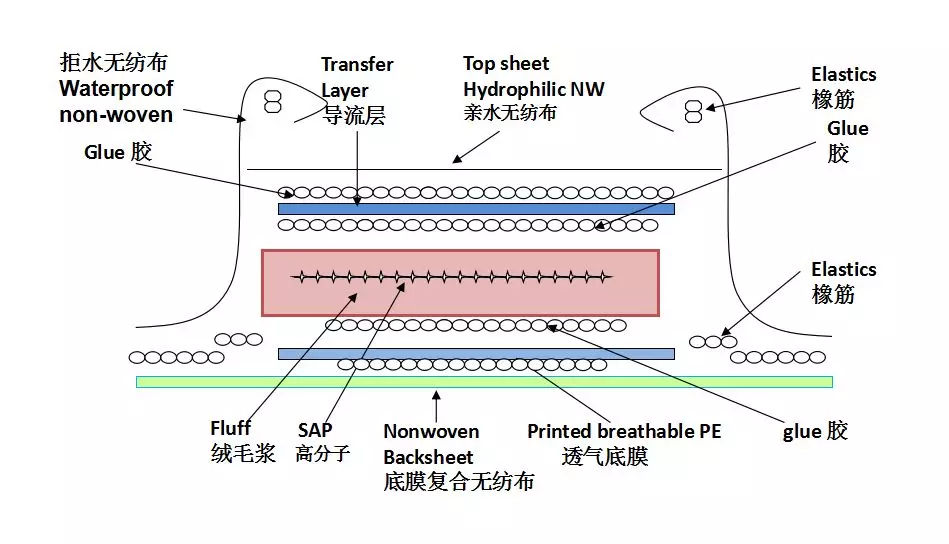
Unlocking the Potential of SAP High Polymer Materials in the Medical Consumables Industry
2024/03/01Unlocking the Potential of SAP High Polymer Materials in the Medical Consumables IndustryIn the ever-evolving landscape of materials science, SAP (Superabsorbent Polymer) has emerged as a remarkable innovation with diverse applications across var...
-

Understanding N95 Respirators: An In-depth Guide
2024/03/01IntroductionThe N95 respirator, a critical piece of personal protective equipment, has garnered significant attention in recent times due to its role in mitigating the spread of airborne diseases. This article provides a comprehensive overview ...
-
The Dental Saliva Ejector: An Essential Tool in Dentistry
2024/03/01Here is the content:Origin and Historical DevelopmentMaterial Characteristics and Key FeaturesApplications and Related Dental ConsumablesOrigin and Historical DevelopmentThe Dental Saliva Ejector, also known as a dental suction, is a vital tool used ...
-

The choice of Isolation gowns raw materials
2024/03/01The choice of Isolation gowns raw materialsDisposable isolation gown is in increasing demand due to the COVID-19 pandemic and it is very important for patient care during infection control efforts.The isolation gown is disposable and protective when ...
-
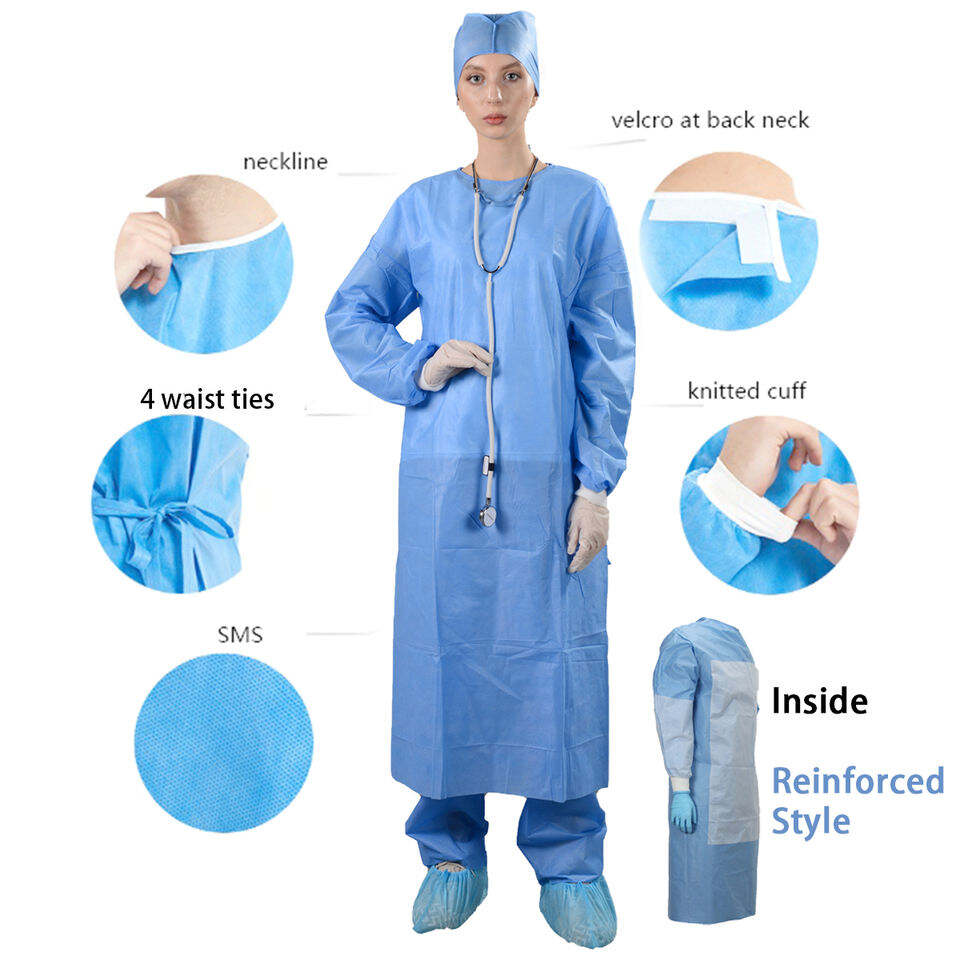
The basic knowledge of surgical gown
2024/03/01The fabric used in the surgical gown belongs to the shielding fabric for medical use, mainly focusing on the barrier performance. Barrier properties include the ability to prevent penetration of liquids and microorganisms. Medical staff in me...
-
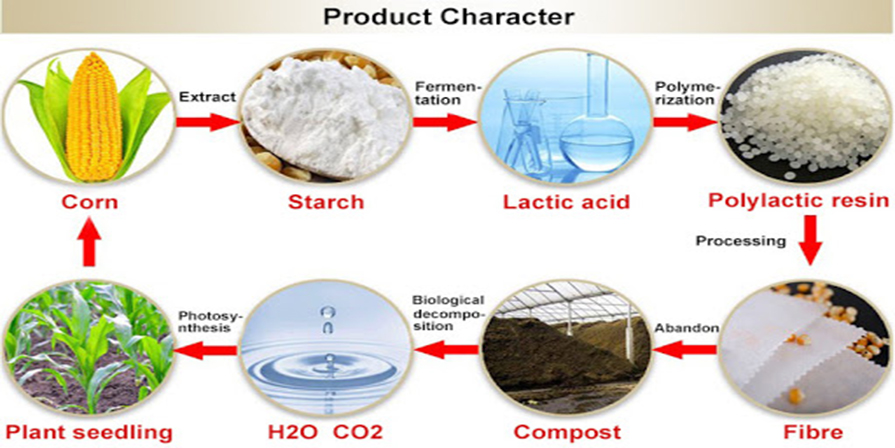
Sustainable Materials (PLA/RPET) Integration in the Disposable Medical Consumables Industry
2024/03/01IntroductionThe disposable medical consumables industry is constantly evolving, driven by advancements in materials and technologies to meet the demands of a rapidly changing healthcare landscape. One significant development in recent years has been ...
-

Reasons for using high-quality medical bed sheet
2024/03/01Medical bed sheets are used to protect a patient's skin from contact with bed linens and other medical equipment. They are perforated to allow air to pass through, and they are made of materials that can be sterilized.The medical bed sheet that is pe...
-

Protective Coveralls: Your Ultimate Guide to International Standards and Suit Selection
2024/03/01IntroductionProtective coveralls play a vital role in safeguarding workers and professionals across various industries. These versatile garments shield wearers from hazardous substances, liquids, and particles, ensuring a safe working environ...
-
PP Coverall: Your Ultimate Protective Solution for All Environments
2024/03/01Introduction:In today's world, personal safety is a paramount concern. Whether you are working in a hazardous industrial environment or dealing with contagious diseases, ensuring your safety is essential. That's where PP Coveralls come into play. In ...
-

Popularization of knowledge about medical bed sheet
2024/03/01The medical bed sheet uses water proof material, which is very comfortable to ues. It is a tool for medical treatment and are suitable for hospitals, beauty salons and other similar facilities. They can be used as the protector of patients wh...
 EN
EN
 AR
AR
 BG
BG
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 HI
HI
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 TL
TL
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 VI
VI
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 GA
GA
 CY
CY
 IS
IS
 LA
LA













